

|
Synthesis of Small Molecule Natural Product-like Libraries |
|
Research Interests
Gold-catalyzed Glycosylation Laboratory (G2L) s.hotha@iiserpune.ac.in +91 20 2590 8015, +91 20 2589 9790 (fax), +91 9823677254
|
|
Discovery of small molecule inhibitors depends largely on the collection of molecules which are diverse and many efforts are been made in the literature for synthesizing libraries which are diverse and natural product-like. A quick comparison of many large collections of small molecules [revealed that , on average, natural products have higher molecular weights, incorporate fewer N-atoms but more O-atoms, and are sterically more complex with number of rings and chiral centers [Feher, M and Schmidt, J. M. J. Chem. Inf. Comput. Sci. 2003, 43, 218-227]. In this realm, we envisioned that carbohydrates would be ideal for synthesizing highly oxygen-rich, multi-cyclic, chiral and architecturally complex small molecule libraries.
We have applied various complexity generating reactions such as Pauson-Khand reaction, 1,3-dipolar cycloaddition, olefin metathesis, enyne metathesis, Diels-Alder reaction and Hashmi's gold catalysed reactions on carbohydrate template to synthesize small molecules [see Diverse Tree]. |
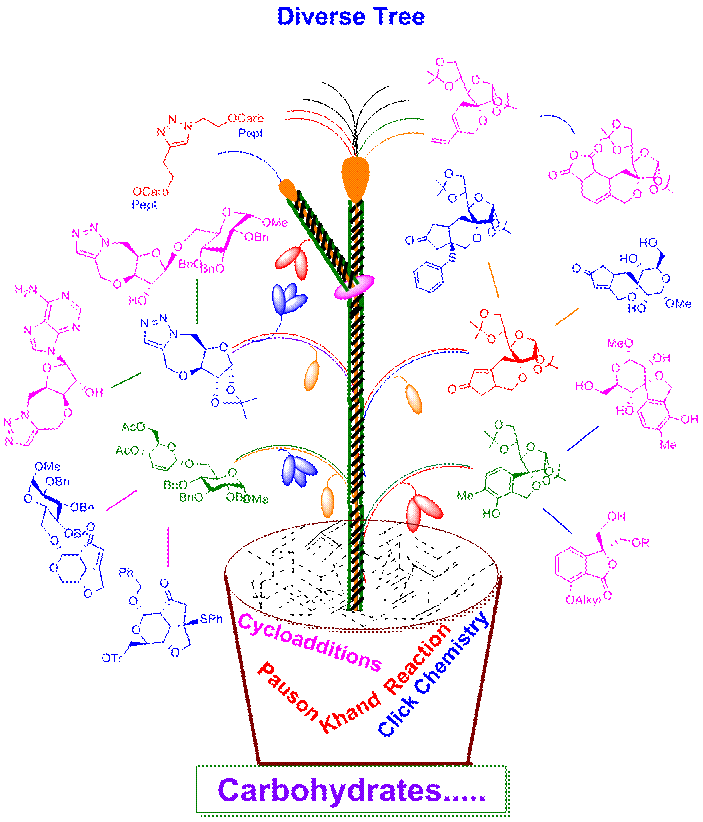
|
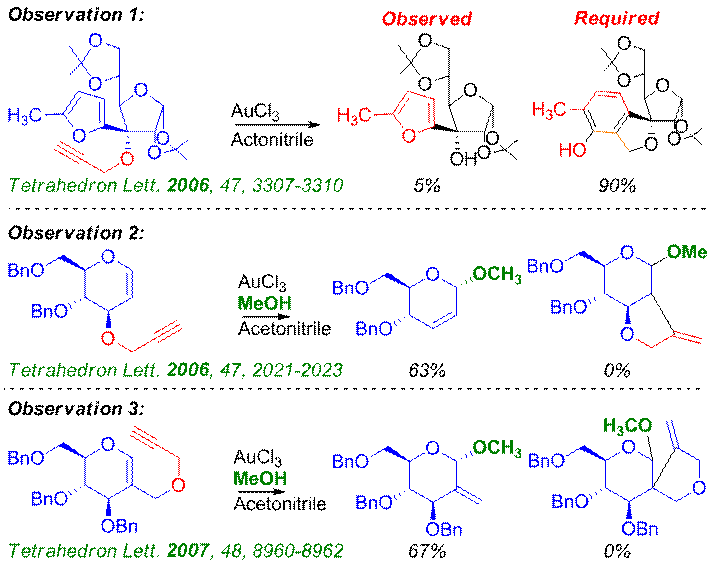
Diversity to Discovery The focus of the group shifted from Diversity Oriented Synthesis to development of glycosyl donor as a result of series of serendipitous observations while generating the Diverse Tree. For example, Hashmi's gold catalysed reaction on monosaccharide resulted in the required isobenzofuran annulation; but, a small amount of propargyl ether deprotection happened. Around the same time, efforts to synthesize fused pyro-furans resulted in the Ferrier-like product and similarly C-2 methylene compounds were obtained instead of spirocyclic compound from propargyoxymethylene glycals which led to the Discovery of Propargyl Glycosides as Glycosyl Donors and 3G. |