lab members working on this project
Current : Soumen Khan, Afsah Hasan
Past members : Naveen Prasad, Shree Harsha TT, Dhanashree Khanale, Farhat Habib, Abhijit Awadhiya.
Evolution of insect hindwing morphology: A comparative genomic analysis of targets of Hox protein Ultrabithorax
The major focus of this study is to understand molecular changes that are associated with the evolution of halteres in Dipterans such as the fruitfly Drosophila melanogaster. We have chosen the honeybee, Apis mellifera, a member of Hymenoptera (which represents first divergence amongst endopterygotes), the beetle Tribolium casteneum a member of Coleoptera (which represents the second divergence that separated them from Lepidopterans and Dipterans) and the Silkmoth, Bombyx mori, a member of Lepidoptera (which represents the third divergence during which Dipterans were separated from other insects) for our comparative studies.
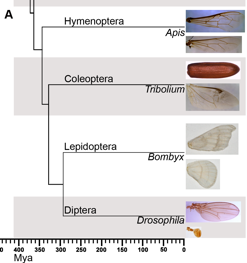
Phylogenetic relationship of four major types of endopterygote insects and their wing morphology. This traces the divergence of 4 major orders of endopterygote insects for nearly 350 million years. Forewing and hindwing morphology of each insect is shown in the panel on the right side.
In Drosophila, the differential development of wing and haltere is dependent on the function of the Hox protein Ultrabithorax (Ubx). We have observed that when over-expressed in transgenic Drosophila, Ubx derived from Apis, Bombyx or Tribolium can induce wing-to-haltere transformations, which are identical to those caused by the over-expression of Drosophila Ubx. It is, therefore, likely that changes in the events downstream of Ubx could be the driving force in modulating differences between forewing and hingwing morphology across all insects. To gain insights into the species-specific events downstream of Ubx, we have identified direct targets of Ubx from Apis and Bombyx by ChIP-seq and compared them with those of Drosophila at the level of both their function and regulation. While the majority of the targets are species-specific, a considerable number of wing-patterning genes are retained as targets of Ubx over the past 300 millions years. Interestingly, most of those are differentially expressed only between wing and haltere in Drosophila and not between forewing and hindwing in Apis or Bombyx. Our observations suggest that in addition to acquisition of new targets, changes in the mode of regulation of earlier targets may have driven the evolution of wing morphology in T3 as a function of Ubx. Detailed bioinformatics and experimental validation of regulatory sequences are being pursued to understand the diversity in Ubx function across the three insect families.
The putative quadrant enhancer of vg of Apis drives the report gene GFP in transgenic Drosophila in a pattern similar to the quadrant enhancer of vg of Drosophila. (A,B) Drosophila vg-Q lacZ wing (A) and haltere (B) discs stained for LacZ (green) and Wg (red). Wg marks the D/V boundary. Drosophila vg-Q lacZ is expressed in non-D/V cells of the wing pouch, but is completely absent from the haltere discs. This transgenic line is reported by Kim et al. (1996. (C,D) Drosophila vg-Q GFP wing (C) and haltere (D) discs stained for GFP (green) and Wg (red). Note, the expression pattern is very similar to vg-Q lacZ. (E,F) Apis vg-Q GFP wing (E) and haltere (F) discs stained for GFP (green) and Wg (red). Note strong GFP expression in both wing and haltere discs. The expression pattern is identical in the two discs and is limited to non-D/V cells of the pouch and in the presumptive hinge. Expression along the A/P boundary is lower, suggestive of quadrant expression pattern similar to the Drosophila vg-Q GFP. (G,H) vg-GAL4/ Apis vg-Q GFP; UAS-UbxDrosophila (G) and vg-GAL4/ Apis vg-Q GFP; UAS-UbxApis (H) wing discs stained for GFP (green) and Wg (red). Note there is no change in the expression pattern of Apis vg-Q GFP. This is contrary to the severe repression observed for Drosophila vg-Q lacZ (Fig. 3J). (I,J) None of the two different mutant versions of Apis vg-Q GFP show any GFP staining in wing or haltere discs suggesting that mutating Adf-1 binding sites to MAD-binding sites may have resulted in complete loss of its activation during wing development. GFP that is seen in I is not nuclear and appears to be non-specific. In Apis vg-Q GFPM1, Adf-1 binding site tggctgccgtcgcgat is replaced with MAD1-binding site (as in the Drosophila genome) gctgcccgccgc. In Apis vg-Q GFPM2, Adf-1 binding site tggctgccgtcgcgat was replaced with MAD1-binding site (as in the Apis genome) gccgtcgc.