Energy Storage
Structure and ion dynamics in lithium and sodium-ion battery electrolytes.
Our group works on computational modeling and simulations on electrolytes for lithium-ion and sodium-ion batteries. We examine properties of soft-solid cocrystalline battery electrolytes in collaboration with experimental groups of Professor Michael Zdilla and Professor Stephanie Wunder (Temple University, Philadelphia, USA). The experimental groups synthesize various soft-solid materials that are lithium or sodium ion salts co-ordinated with organic solvents such as N,N-dimethylformamide (DMF), Adiponitrile, etc.
A) Lithium-ion battery electrolytes
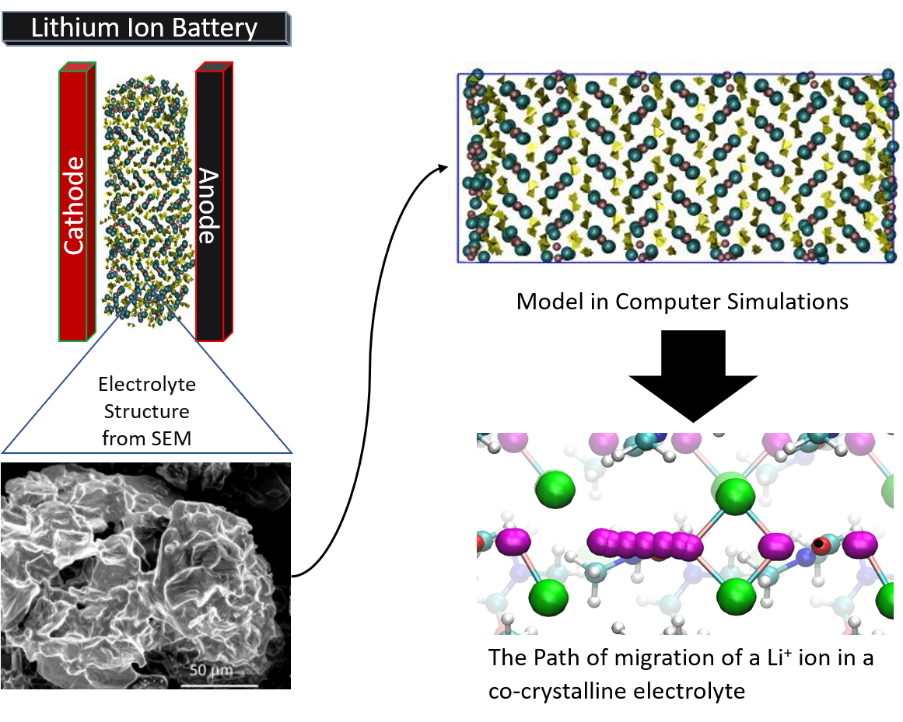
Further reading: P. Prakash, J. Aguirre, M. Van Vliet, P. Chinnam, D. Dikin, M. Zdilla, S. Wunder, A. Venkatnathan, Unravelling the structural and dynamical complexity of the equilibrium liquid grain-binding layer in highly conductive organic crystalline electrolytes, J. Mater. Chem. A. 6, 4394 (2018).
B) Sodium-ion battery electrolytes
Abundant and inexpensive in comparison to lithium, sodium is often posited as an attractive alternative to lithium-ion batteries. The changing temperature or pressure, a soft solid material such as sodium perchlorate could reversibly release or absorb an organic solvent (dimethylformamide or DMF). To validate experimental findings, the group modelled the mechanism of DMF-sodium perchlorate conversion and stimuli response with changes in temperature and pressure. In a subsequent paper, in Journal of Physical Chemistry C the group applied quantum chemistry calculations and observed several ion conduction pathways in the same material before and after the stoichiometric conversion. The team demonstrated the role of the solvent and anions in sodium-ion mobility.
© 2021 The Author(s). Published by the Royal Society of Chemistry Chem. Sci., 2021, 12, 5574–5581
Prabhat Prakash, Ardhra Shylendran, Birane Fall, Michael J. Zdilla, Stephanie L. Wunder, Arun Venkatnathan, The mechanism of ion conduction and dynamics in tris(N,N-dimethylformamide) perchloratosodium solid electrolytes, J. Phys. Chem. C, 126 (10), 4744, 2022.
As an alternative to solid materials, the Venkatnathan group also investigated a diglyme-based liquid sodium-ion electrolyte. The team examined the effect of ionic concentrations and temperature on ion-ion/solvent interactions and ion dynamics.
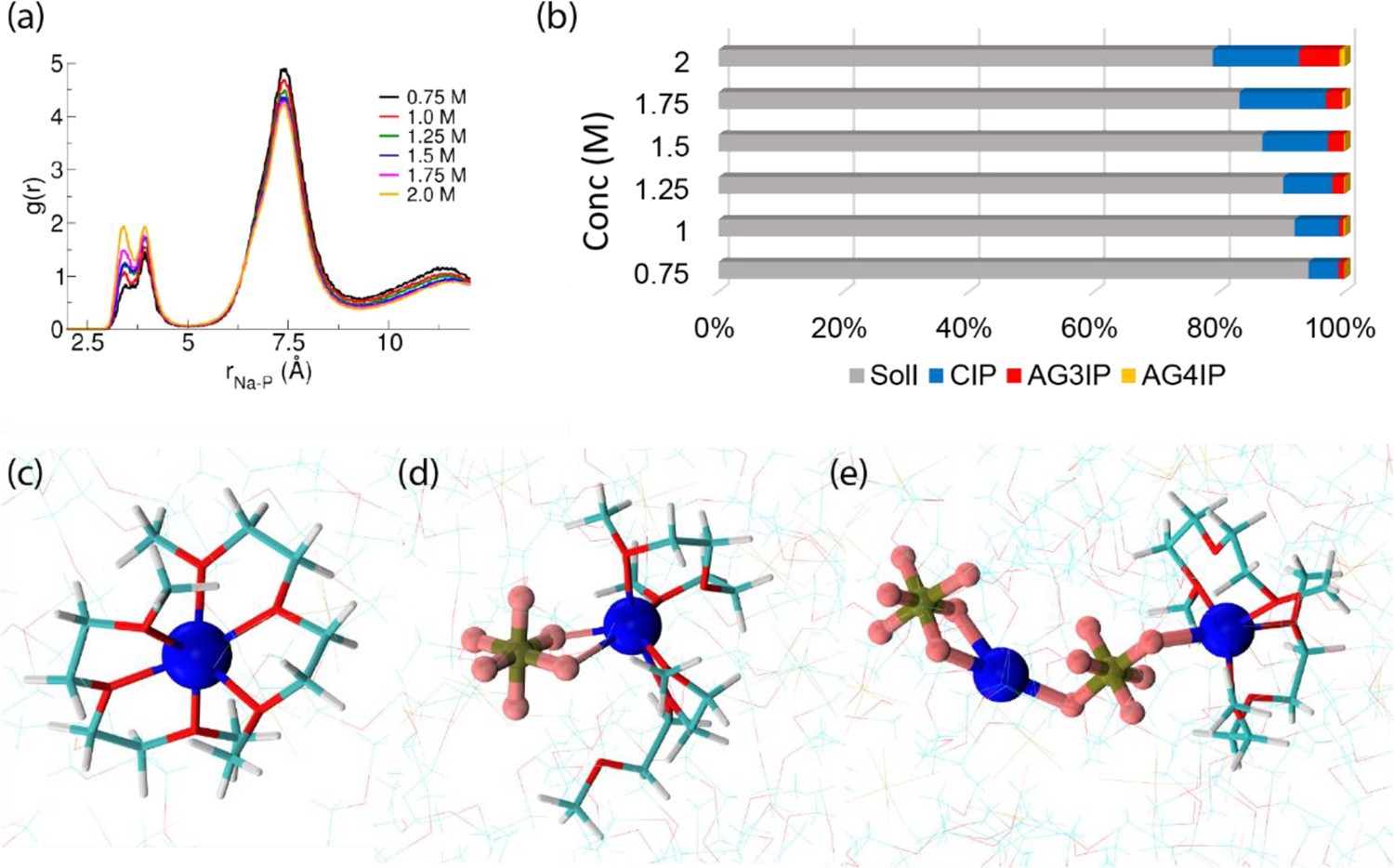
Copyright: American Chemical Society 2022 J. Phys. Chem. B 2022, 126, 10, 2119–2129
