|
Research |

|
Welcome to Polymers & Biomaterials Research Group |
|
Our research team is comprised of humble, extremely hardworking, and highly motivated smart folks who have joined together to learn the elegance of macromolecular (polymer) systems in biomaterial science. The brief account of our group work is summarized under various topics. Þ Eco-friendly Synthesis of Biodegradable Polymers from L-Amino acid Resources Þ Linear, Star, and Brush-type Biodegradable Block Copolymers for Drug Delivery Þ Stimuli-responsive Polysaccharide Polymersomes Þ FRET Bioprobes & Cellular Bio-imaging Þ Antimicrobial Polymers
A. Solvent Free (Melt Polycondensation) Synthesis of L-Amino acid based Biodegradable Polymers
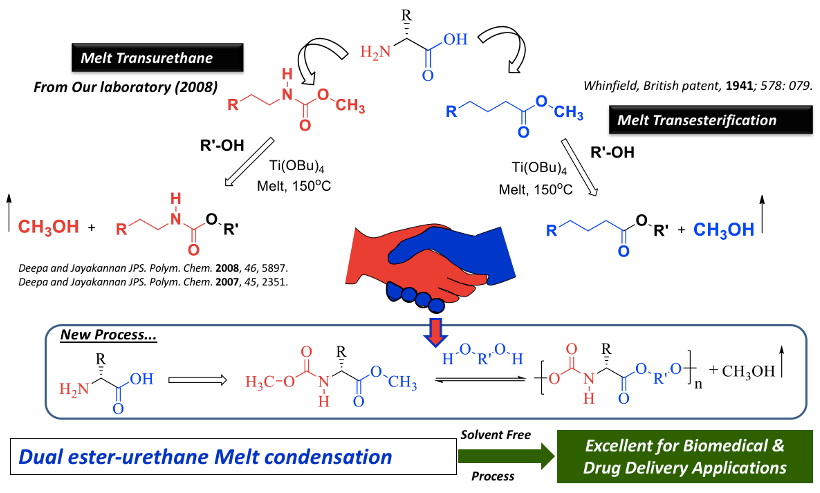
Developments of biodegradable polymeric materials by eco-friendly synthetic methodologies are important task for their long term-impact in biomedical applications. Melt polycondensation approach in polymer synthesis is one of the elegant methodologies that could be expanded from the laboratory process to large scale industrial production. In this process, the organic molecules (monomers) were melted at high temperature under solvent free environments and subjected to polymerization in the presence of suitable catalyst. The resultant polymer can be directly processed into desired consumer or industrial products or biomedical devices. A decade ago, in an adventure to make polyurethanes via non-toxic and isocyanate-free synthetic route, we had invented a melt transurethane process for thermoplastic polyurethanes under solvent melt-process (Deepa et al. 2007 and 2008). By cleverly merging this process with the transesterification reaction; a unique dual ester-urethane melt polycondensation chemistry was developed for L-Amino acid bio-resources (Anantharaj et al., 2012). In this process, L-amino acids were modified into ester-urethane bi-functional monomers and they were subjected to polymerization with commercial diols to produce semi-crystalline or amorphous poly(ester-urethane)s (see Chart-1).
Chart-A1: Development of Melt Polycondensation strategy for L-Amino acid Resources
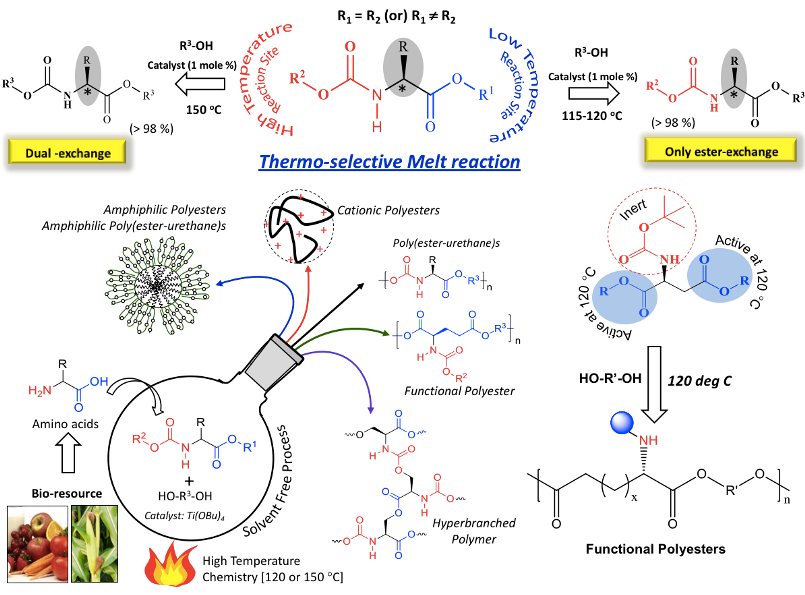
These L-amino acid multi-functional monomers were found to be highly thermo-selective for their reaction with alcohols (or diols). The ester-functionality of the monomer underwent selective transesterification with alcohols in the presence of Ti-catalyst at low temperature (at 120 deg C). At this temperature, the urethane part (carbamate) was completely inert. At higher temperatures (equivalent or above 150 deg C), this selectivity was lost which resulted in the occurrence of simultaneous dual ester-urethane reaction (Anantharaj et al., 2015 and 2016). This enabled us to develop thermo-selective polycondensation approach for multi-functional L-amino acid resources to make diverse polymer products such as amine-functionalized polyesters and hyperbranched poly(ester-urethane)s (Rajendra et al., 2015) and so on so forth. These new polymer structures were not accessible by other synthetic methodologies (see Chart-A2).
Chart-A2: Non-peptide polymers developed in the laboratory based on L-Amino acid Resources
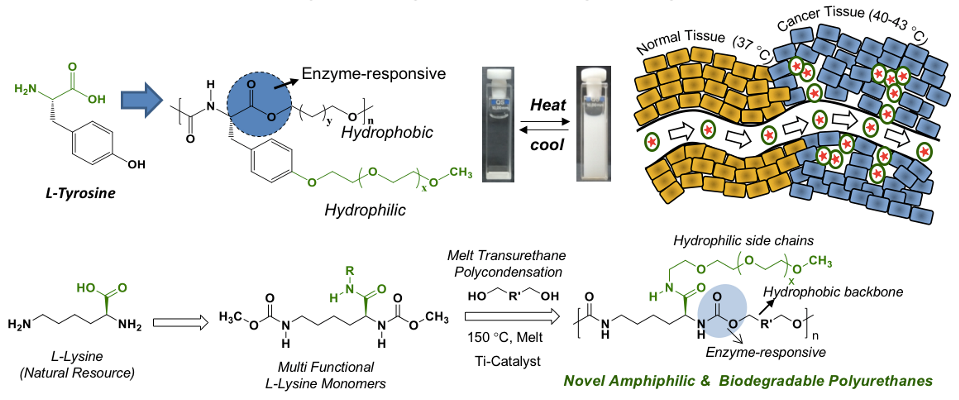
Melt polycondensation approach was further expanded to polyurethane from L-amino acid resources, more exclusively based on L-lysine bio-resources (Dheeraj et al. 2019). Thermo-responsive amphiphilic polymers were accomplished by appropriately optimizing the hydrophilic-hydrophobic balance in the polymer geometry. These thermos-responsive polymers from L-tyrosine and L-lysine polymers exhibited highly selective drug delivery capabilities at cancer tissue temperature (42 deg C) while retaining their intactness at body temperature (37 deg C) (Rajendra et al. Biomacromolecules 2018). These polymers could have long-term impact on biomedical application wherein thermo-responsiveness is a crucial parameter for a particular action (see Chart-3).
Chart-A3: Development of thermo-responsive polyurethanes and poly(ester-urethane)s
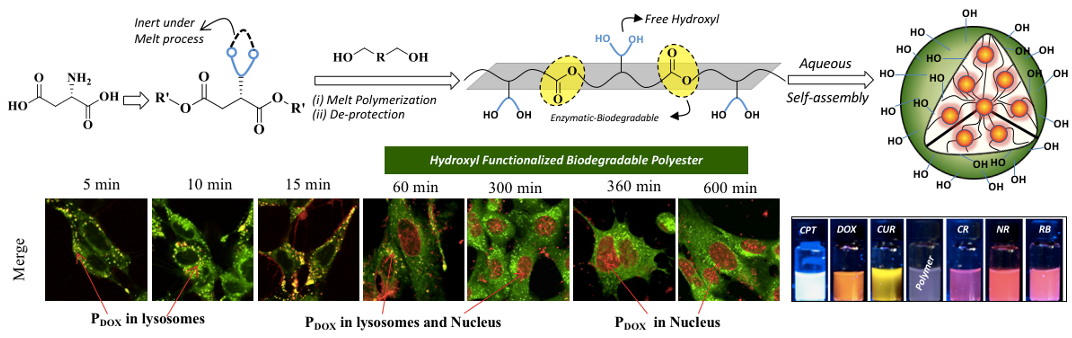
Recently, we have reported the developments of novel hydroxyl-functionalized amphiphilic polyesters based on L-amino acid bio-resources and explore their nano-assemblies as biomaterials. L-Aspartic acid was converted into acetal-masked multi-functional dicarboxylic ester monomer which was subjected to melt transesterification polycondensation with commercial diols to produce acetal-functionalized polyesters. Acid-catalyzed post-polymerization de-protection of these hydrophobic acetal-polyesters produced new classes of amphiphilic hydroxyl-functionalized polyesters. This process is shown in Chart-A4 (Sonashree et al. 2020). Hydroxyl-functionalized polyesters exhibited superior encapsulation capabilities to load wide ranges of both water-soluble and water-insoluble anticancer drugs like doxorubicin (DOX), camptothecin (CPT) and curcumin (CUR) and fluorophores such as Nile red (NR), Rose Bengal (RB) and Congo red (CR). Lysotracker assisted live-cell confocal microscopy studies confirmed the co-localization of the polymer nanoparticles in the lysosomal compartments and provide direct evidence for the enzymatic-biodegradation of the polyester nano-carriers (see Chart-A4).
Chart-A4: Hydroxyl Functionalized Polyesters and their Drug (DOX) Delivering Capabilities in Live Cells
The above design strategy opens up new opportunities for biodegradable polymer platform based on L-amino acid bio-resources. Further progress in the biomaterials development would be updated soon.
B. Linear, Star, and Brush-type Biodegradable Block Copolymers
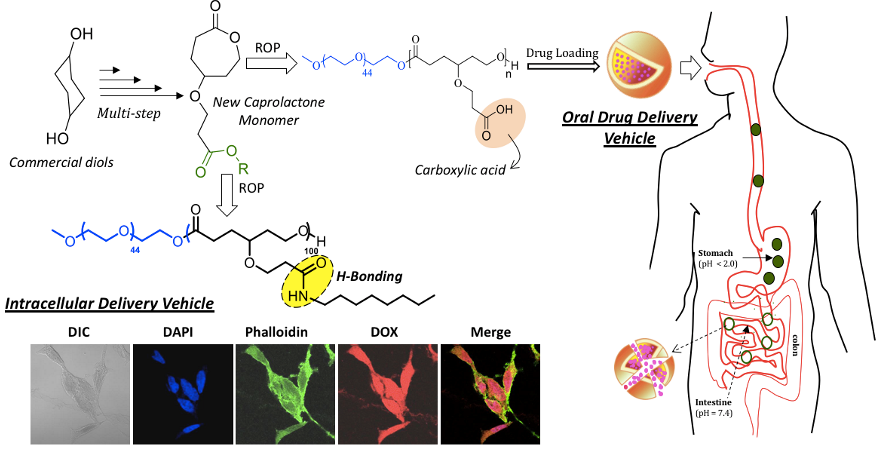
Aliphatic polyesters are considered to be holy-grails in the biomaterial arena due to their nearly 100 % enzymatic-biodegradability under physiological conditions. Commercial aliphatic polyesters such as poly(L-lactide)s (PLLA) and polycaprolactone (PCL) are made by the ring opening polymerization (ROP) methodology. About 10 years back, we initiated work in ROP and our first attempt was to explore the unknown carboxylic substituted PCL based biodegradable polymers for drug delivery. Success in ROP process is highly dependent on cleverly designing new monomers and its applicability to make unexplored macromolecular architectures. 1,4-Cyclohexane diol was employed as starting material to make new carboxylic acid functionalized caprolactone monomer that produced pH responsive block copolymers for delivery of the drugs under tightly regulated gastrointestinal tract (Bapurao et al. 2013). These diblock copolymers were programmed for enzymatic-biodegradability by structural engineering of the core by hydrogen bonding secondary interaction. The enzyme-stimuli provided a handle to tailor make the slow and fast degradation at the cellular level (Bapurao et al. 2016).
Chart-B1: Carboxylic Functionalized PCL Block Copolymers
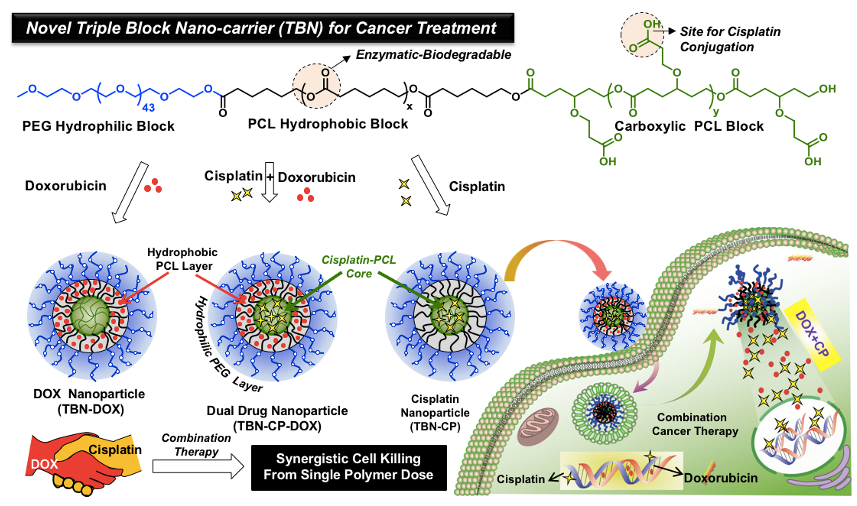
These carboxylic block copolymers were successfully employed for the chemical conjugation of metal-based anticancer drug cisplatin to create well defined Pt-produrg core-shell nano-particles of 80 to 200 nm sizes depending upon the segmental lengths (Bapurao et al. 2015). This cisplatin-prodrug is very active against the GSH-assisted drug detoxification, pretty stable and efficient in suppressing the breast cancer cell growth. Combination therapy is also accomplished for the dual delivery of doxorubicin and cisplatin together in the single PCL block copolymer platform (see Chart-B2, Bapurao et al. 2016).
Chart-B2: PCL-based Pt-prodrugs for Combination Therapy
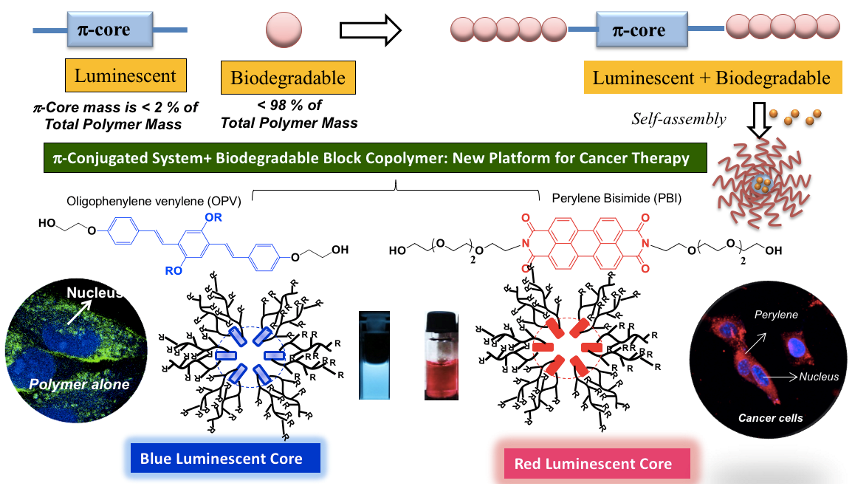
Blue and red-Fluorescent Π-conjugated fluorophores based on oligo-phenylenevinylene and perylenebisimides were tailor made as initiator for the ROP of PCL systems to produce biodegradable and fluorescent block copolymer nano-assemblies. These fluorescent probes were employed as theranostic probes and also for bio-imaging applications at the cellular level (see Chart-B3) (Bhagyashree et al. 2016 and 2019).
Chart-B3: Fluorescent PCL Block Copolymers for Biomedical Application
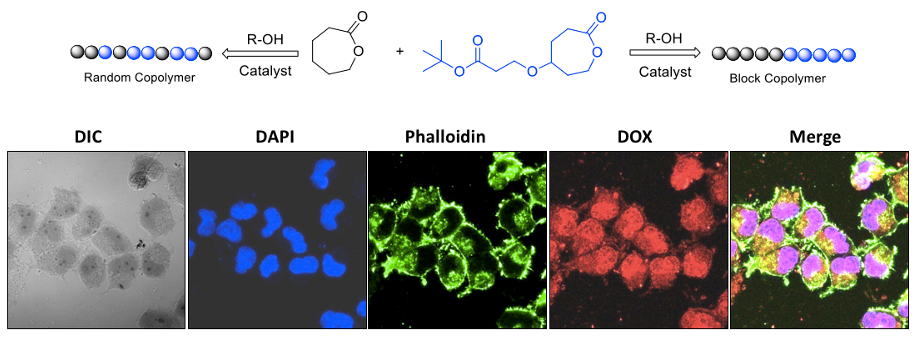
Polymer topology plays a major role in the aqueous self-assembly of nano-carriers in drug delivery applications. To study the role of polymer topology on the enzymatic-degradation of PCL based nano-scaffolds under physiological conditions is very crucial for the long-term development of fully aliphatic polyesters for biomedical applications. To address this important problem; we have engineered new classes of fully biodegradable block and random copolymer macromolecular architectures based on hydrophilic carboxylic functional polycaprolactone (PCL) and hydrophobic polycaprolactone segments. These newly designed block and random copolymers are readily programmable with respect to their polymer topology for enzymatic-digestion at the intracellular compartments in breast and cervical cancer cell lines (see chart-B4, Mehak et al. 2016).
Chart-B4: Topology Control in PCL Block Copolymers for Drug Delivery Currently efforts have been taken to expand the PCL block copolymer approach to star and the brush type copolymers and the detail will be updated soon. Efforts has also been taken to expand the approach to polypeptides. These details will be added soon.
C. Stimuli-responsive Polysaccharide Polymersomes
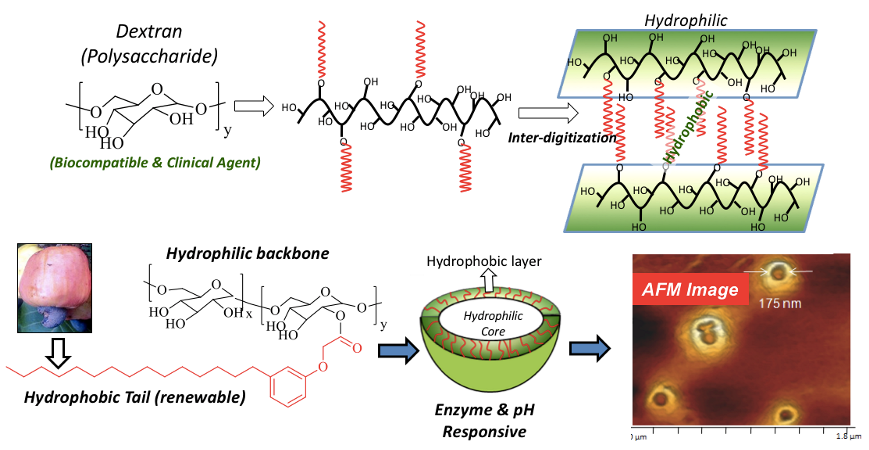
Polymersomes (or polymer vesicles) are very unique nano-scaffolds because of their inherent and well-defined compartmentalization of hydrophilic core and hydrophobic layer for loading water-soluble and water-insoluble cargoes, respectively. Further polymersomes largely exist as single or isolated nano-objects (like dendrimers) and they are almost unperturbed to disassembly by the concentration gradient unlike other aggregated nano-species such as micelles or nanoparticles. These unique features make polymersomes (or polymer vesicles) as a distinct nano-carriers in biomedical applications. Our research group has engineered bio-resourced based polymersomes by conjugating plant-based hydrophobic unit 3-pentadecyl phenol (PDP, from cashew nut shell liquid) as vesicular directing component on the water soluble polysaccharide-dextran to bring the appropriate geometry for polymersome self-assembly in water. Single Crystal analysis is clearly evident of the strong inter-digitization of the hydrophobic units for formation of vesicular nano-assemblies (see Chart-C1, Pramod et al. 2012, Smita et al. 2012).
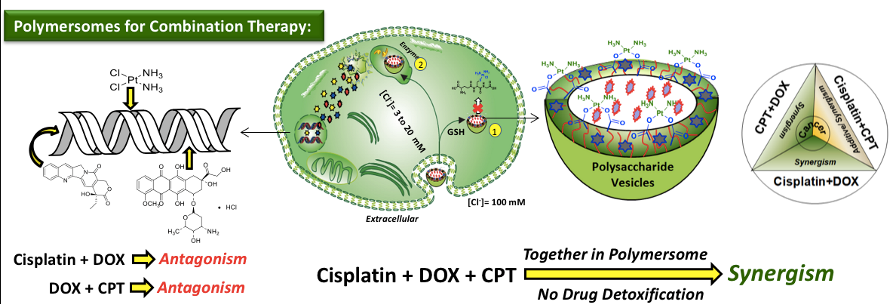
Chart-C1: Polysaccharide Polymersomes The PDP hydrophobic handle is versatile to carry enzyme, pH and GSH responsive polymersomes depending upon the choice of the chemical linkages that are chosen to programme their stimuli-responsive delivery at the cellular level. The aliphatic ester linkage connecting the hydrophobic tail with dextran was found to be cleaved by lysosomal-esterase enzyme under physiological conditions for fast release of loaded cargoes (Pramod et al. 2014). Acid labile benzylic imine linkage was employed to cleave the polymersomes under acidic endosomal pH conditions (Pramod et al. 2005). The disulphide (-S-S-) chemical linkages facilitated the GSH-resposive delivery of polymersomes (Nilesh et al. 2018). In vitro studies revealed these polymersomes are very stable under physoogical conditions (pH = 7.4 at 37 deg C) and exhibited 80-90 % of drug release under intracellular stimuli-responsiveness (see Chart-C2). Carboxylic functionalized dextran was tailor-made for the chemical conjugation of cisplatin to produce cisplatin-stitched polysaccharide nanovesicles. Water soluble DNA-intercalating drug doxorubicin.HCl (DOX) and water insoluble topoisomerase type I inhibitor drug camptothecin (CPT) were encapsulated in these vesicles to produce dual or triple drug loaded vesicular nano-carrier. This unique cisplatin, DOX and CPT triple drugs loaded dextran vesicles were stable in aqueous medium and the vesicular geometry acted as a shield for Pt-polymer drug conjugate against glutathione (GSH) detoxification under physiological conditions (Nilesh et al. 2017). In vitro cytotoxicity studies revealed that free cisplatin was highly detoxified by the GSH in breast cancer cells whereas the enhanced stability of Pt-stitched dextran vesicle against GSH facilitated ~ 99 % cell killing in breast cancer cells (see Chart-C2).
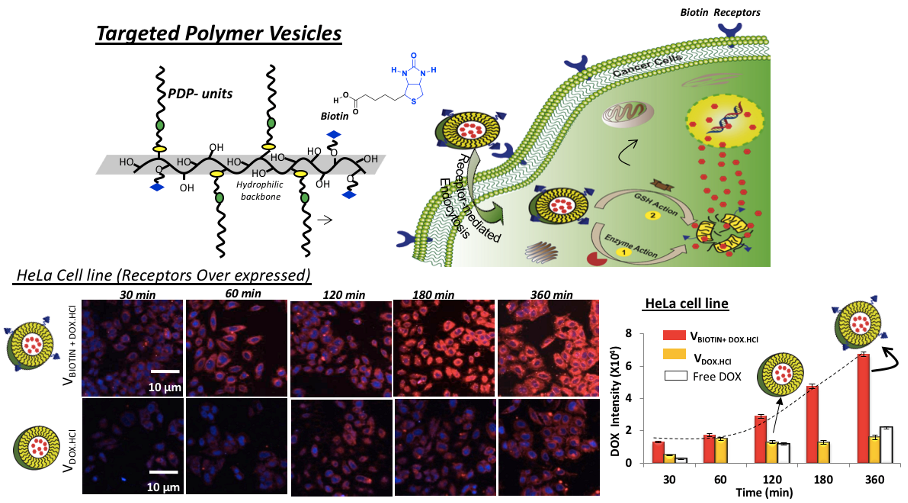
Chart-C2: Polymersomes for combination therapy of Triple drugs Further, receptor-mediated endocytosis was accomplished using biotin-tagged polymersomes. Time-dependent cellular uptake studies revealed that the biotin-receptors, which are over expressed on cervical cancer cells (HeLa), exhibited larger drug accumulation through receptor-assisted endocytosis process. This process enabled the delivery of higher amount of DOX and significantly enhanced the killing in cancer cells (HeLa) compared to wild-type mouse embryonic fibroblast cells (WT-MEF, normal cells) (see chart-C3, Nilesh et al. 2018).
Chart-C3: Receptor mediated Endocytosis in Targeted Delivery Collaboration work with Prof. Nagaraj Balasubramainan Group: Polymersome platform is used for the first time to deliver and differentially inhibit Aurora kinase A (AURK-A) by successfully administering MLN8237 to cancer cells in 2D and 3D microenvironments. In breast cancer (MCF 7) cells, the drug containing polymersome inhibits AURKA significantly better than the free drug at low concentrations (0.02 to 0.04mM). This design ensures that the drugs in polymersome at these concentrations can specifically inhibit up to 94% of endogenous AURK-A without affecting AURK-B. This targeting of AURKA causes the downstream differential inhibition of active RalA (but not RalB). Free MLN8237 at similar concentrations and conditions failed to affect Ral-A activation. Drug in polymersome mediated inhibition of RalA, in turn, disrupts the anchorage-independent growth of MCF 7 cells supporting a role for the AURKA-RalA crosstalk in mediating the same. These studies not only identify the polysaccharide nanovesicle to be an improved way to efficiently deliver low concentrations of MLN8237 to inhibit AURK-A but in doing so also help reveal a role for AURKA and its crosstalk with RalA in anchorage-independent growth of MCF 7 cells (Siddhi et al. 2018). Further, Caveolae-mediated endocytosis was also studied. Polymersomes were preferentially taken by the breast cancer MCF 7 cells to enhance the therapeutic effects. Endocytosis of this nano-vesicle in caveolae lacking (Cav-1 -/- ) MEFs was seen to be better. This differential uptake is also seen in caveolin-1 (and hence caveolae lacking) breast cancer (MCF7) and colon cancer (DLD1) cells. The dual loaded polysaccharide nano-vesicles containing DOX and CPT act synergistically to promote killing of cancer cells. When administered as a cocktail having more CPT than DOX, synergistic killing of cancer cells was enhanced the most suggesting that a cocktail of these drugs could potentially be the best candidate for delivering multiple anticancer drugs with the best efficacy. The results provide significant new insights into polysaccharide loaded dual drug action of DOX:CPT drug combination in the breast and colon cancer cells (Pramod et al. 2014). Currently efforts have been taken to make fluorescent polymersomes as theranostic probes and these details will be added soon.
D. FRET Bioprobes for Cellular Bio-imaging
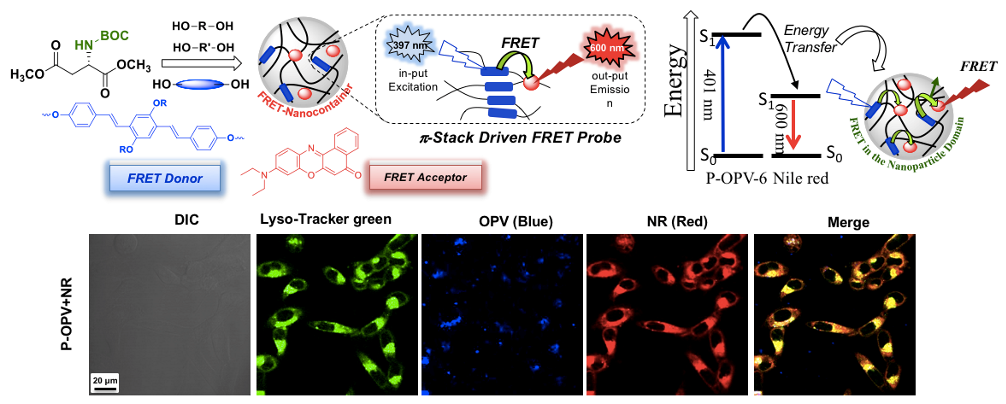
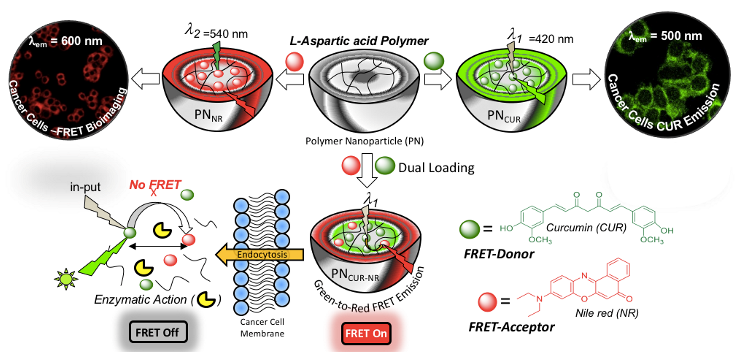
Fluorescence resonance energy transfer (FRET) bioprobes are excellent system to study large number of biological probes including the real-time drug delivery aspects at the intracellular compartments. Fluorescent-tagged polymer nano-assemblies can be employed as FRET donor (or acceptor) along with the fluorescent anticancer drugs as FRET acceptor (or donor), vice versa, and bring their association in less than 100 Angstrom distance (or 10 nm) to enable them as FRRT probes. We have designed and developed oligo-phenylenevinylene tagged biodegradable polymer based on L-amino acid polyesters and carboxylic PCL block copolymers (Bhagyashree et al. 2018). The encapsulation of water insoluble Nile Red (FRET acceptor) in the polymer nanoparticle cavity enabled the formation of OPV-NR FRET donor-acceptor pairs. These newly designed polymer probes are biodegradable exclusively at the intracellular level by wide ranges of lysosome-enzymes. Thus the probes would readily biodegrade by enzymes once the imaging job is done at the cellular level which is desirable for their excretion from the point of target. The FRET probe was employed as colour-tunable biomarker and the proof-of-concept was demonstrated in vitro at the intracellular compartments of cells (see Chart-D1, Sonashree et al. 2017).
Chart-D1: Enzyme-responsive polymer FRET Probes Biodegradable polymer nano-assemblies have the capabilities to function as a FRET probe provides additional advantage. Once the bio-imaging or therapeutic job is done; they can completely be chopped into smaller chains and could be readily excreted from the biological system. Hence it would be appropriate to build theranostic FRET nano-carriers based on biodegradable platform from L-amino acid based resources. Amphiphilic L-aspartic acid polyester nano-carrier was used to construct FRET pair between curcumin drug and Nile red fluorophores and the proof-of-concept has been demonstrated in live cell breast cancer cell bio-imaging. The theranostic FRET probe was found to be very stable at extracellular environment whereas the aliphatic polyester backbone underwent lysosomal enzymatic-biodegradation at the intracellular compartments to deliver the cargoes. As a result, the theranostic FRET probe function as turn-On at the extracellular level and became turn-Off at the intracellular level. Live cell confocal microscopy studies and selective photoexcitation experiments in the confocal microscope were carried out to study the FRET probe action in cancer cells and time-dependent FRET imaging further directly supported the occurrence of the FRET at the cellular level (see Chart-D2, Sonashree et al. 2019).
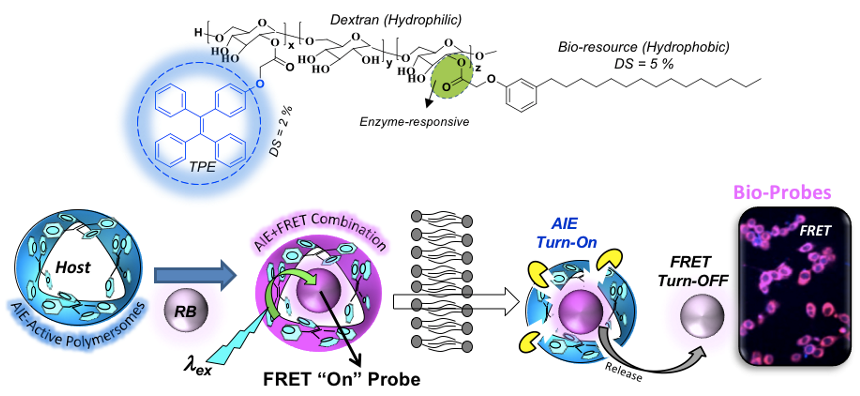
Chart-D2: Theranostic FRET Probes Recently, the aggregation induced emission (AIE) concept is introduced in the polysaccharide polymersome to combine two photophysical processes AIE and FRET in single nano-system. AIE and FRET concept concepts were exploited between polymersome donor and biocompatible fluorophore acceptors such as water soluble Rose Bengal (RB) and water insoluble Nile red (NR) to construct intracellular enzyme-responsive FRET probe. The selective photoexcitation of the TPE chromophore enabled the FRET process between TPE donor and RB (or NR) acceptor molecule in < 50 Angstrom Forster distance afforded by the polymersome nano-assemblies (Nilesh and Mishika, 2021 ). In vitro release studies revealed that the FRET probe was very stable at the extracellular condition and it exclusively underwent lysosomal esterase enzymatic-biodegradation at the intracellular compartments to release the RB. The proof-of-concept was established in live cancer cells by selective photo excitation of donor/acceptor in confocal microscope to visualize and quantify the real-time delivering capabilities in cancer live cells (see Chart-D3).
Chart-D3: AIE-driven Polysaccharide Polymersome FRET probe
More efforts in topic will be updated soon.
E. Cationic Antimicrobial Polymers
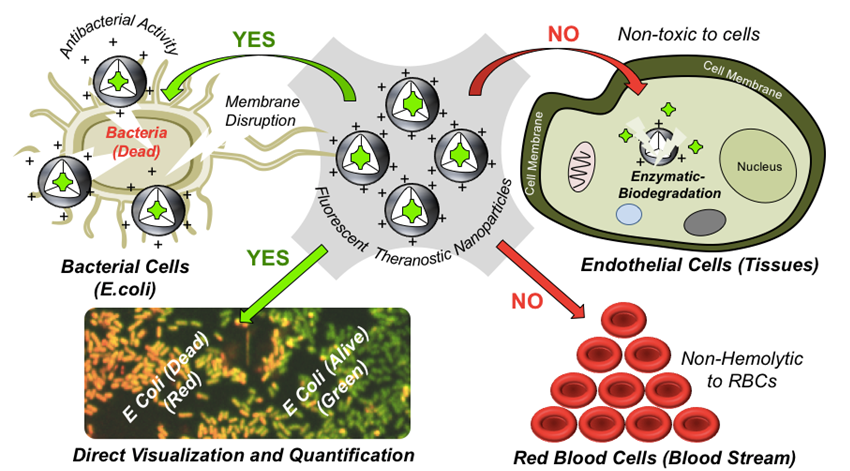
Treatment of infectious diseases caused by bacteria and virus are emerging as the major challenges in addressing the healthcare issues and society at the large. Continually increasing multi-drug resistance and steadfast mutations of microorganisms severely obstruct the treatment with existing drugs or antibiotics, and this also further limits the existing diagnostic methods in the fast detection of infectious diseases. This emphasizes that the next generation candidates are essential to be smart multitasking probes having capability to exhibit both therapeutics and diagnostics together (theranostics) in single dose of drug administration. It is important to realise that the probe molecules come in direct contact with the healthy tissues (or cells), proteins and red blood cells (RBCs) while testing their activity which further introduce unwanted toxicity issues. Therefore, the incorporation of biodegradation property is essential component in theranostic probe design for safer biomedical applications especially in treating the bacterial infections. To address this important problem, recently, we reported one of the first attempts in designing new biodegradable theranostic antibacterial nanoprobes in enzymatic-biodegradable cationic polycaprolactone (PCL) platform. The theranostic nanoprobe was successfully employed for direct visualization and qualitative determination of bactericidal activity by the newly developed fluorescent assay methodology which could distinguish live and dead bacteria in real case scenario. The new approach reports new biodegradable antibacterial polymer (as drug) as well as fluorescent imaging probe to real-time visualization and treatment of infections together in single platform (see Chart-E1, Ruma et al. 2020).
Chart-E1: Development of New cationic PCL polymer platform for Antimicrobial Research Currently efforts have taken to make fluorescent-tagged cationic polymers to study the mechanistic aspects in detail and these details will be added soon.
|